Naturally available uranium consists of mainly uranium-238 isotope (about 99.3% of the natural uranium is U-238 isotope, 0.7% is U-235 isotope and trace amount of U-234). Among the three naturally available isotopes of uranium, only U-235 is fissile material and can sustain chain reaction as it offers higher cross-section towards thermal neutron. So only U-235 can be used as fuel in nuclear reactors, but its availability in the natural uranium is just about 0.7%. Due to lack of natural availability, the proportion of U-235 is required to increase artificially to prepare nuclear fuel. The process of increasing the proportion of U-235 in a uranium mass is called enrichment. Typically, thermal reactors of the nuclear power plant require 3 – 5% enriched uranium, fast reactors require 15 – 20% enriched uranium, while weapon grade fuel require above 90% enriched uranium. There are several techniques for enriching the uranium fuel, some of them include (i) Gaseous diffusion, (ii) Gas centrifuge, (iii) Laser based processes – AVLIS, MLIS and SILEX, (iv) Aerodynamic enrichment, (v) Electromagnetic separation, (vi) Chemical separation, (vii) Plasma separation, etc. In 20th Century, the gaseous diffusion process was the most popular method for uranium enrichment; however, it gradually became obsolete with the development of cheaper and less time consuming processes. In today’s world, most of the separations are carried out by Gas Centrifuge process, while the other processes are either economically not viable or still under development.
Gaseous diffusion process works based on the Graham’s Law of effusion (the rate of effusion of a gaseous substance is inversely proportional to the square root of its molecular mass). Metallic uranium is first converted into uranium hexafluoride (UF6) gas that has natural concentrations of U-235 and U-238. It is then kept in a large cascade under pressure and the lighter isotope is allowed to pass via a semi-permeable membrane. U-235 being about 0.85% lighter than U-238, the former one passes the semi-permeable membrane more rapidly compared to the later one. Thus UF6 with slightly higher concentration of U-235 can be obtained inside the membrane chamber. Multi-stage separation can ultimately provide significantly enriched UF6. This enriched UF6 can then be converted into uranium dioxide (UO2) to use as nuclear fuel. On the other hand. Gas Centrifuge process is also based on the slight mass difference among U-235 and U-238; however, here a rotating centrifuge is employed rather than a semi-permeable membrane. The UF6 gas is first introduced in the rotating centrifuge. U-238 being heavier experiences higher centripetal acceleration and thus flows towards the outer wall of the centrifuge, whereas U-235 remains close to the axis of the centrifuge. Accordingly, enriched uranium can be extracted by sucking out the UF6 gas from the centrifuge axis. This enriched UF6 gas is then converted to uranium dioxide (UO2) as usual to use as nuclear fuel. Various similarities and differences between gaseous diffusion and gas centrifuge processes for enriching uranium are given below.
Similarities between gaseous diffusion and gas centrifuge
- Working principle of both the processes is based on the slight mass difference between U-235 and U-238 isotopes. U-235 isotope is about 1.26% lighter than U-238 isotope.
- Both the processes require uranium hexafluoride (UF6) gas for separation. No separation is possible from the metallic uranium gas. Thus the oxide of uranium (U3O8, also called yellow cake) is first chemically converted to uranium hexafluoride (UF6) gas. Then separation is carried out. Notably, 235UF6 gas is only about 0.85% lighter than 238UF6 gas, even though the metallic U-235 is about 1.26% lighter than U-238. The enriched UF6 gas is once again converted to uranium dioxide (UO2) and sintered to rod or pallet to use as fuel.
- None of the process can give highly enriched uranium in single stage. Thus multiple stages are require to obtain substantially enriched uranium.
Differences between gaseous diffusion and gas centrifuge
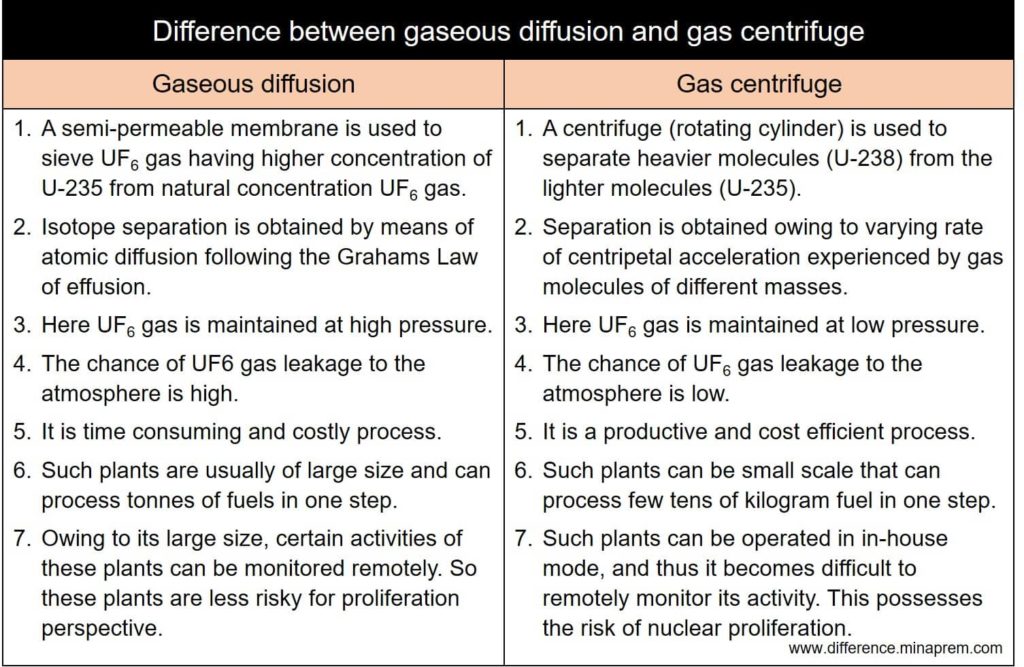
| Gaseous diffusion | Gas centrifuge |
|---|---|
| A semi-permeable membrane is used to sieve UF6 gas having higher concentration of U-235 from the natural concentration UF6 gas. | A centrifuge (rotating cylinder) is used to separate heavier molecules (U-238) from the lighter molecules (U-235). |
| Isotope separation is obtained by means of atomic diffusion following the Grahams Law of effusion (U-235, being the lighter molecule, passes the membrane more rapidly). | This technique is not based on the Grahams Law; rather separation is obtained owing to varying rate of centripetal acceleration experienced by gas molecules of different masses. U-235, being the lighter molecule, remains close to the centre of the centrifuge. |
| Enriched uranium is obtained inside the membrane enclosure, while depleted uranium is obtained outside the enclosure. | Enriched uranium is obtained close to the axis of the centrifuge, while depleted uranium is obtained towards the wall of the centrifuge. |
| Here the UF6 gas is maintained at high pressure (higher than atmospheric pressure). | Here the UF6 gas is maintained at low pressure (usually lower than atmospheric pressure). |
| The chance of UF6 gas leakage to the atmosphere is high. Thus the process is inherently associated with the risk of spreading radioactive elements in the air. | The chance of UF6 gas leakage to the atmosphere is low (as the gas in the pipeline is maintained at a pressure lower than atmospheric pressure, so any leakage will lead to the inward flow of air into the pipeline). |
| Gaseous diffusion is time consuming and costly process. | Gas centrifuge is a productive and cost efficient process. |
| Earlier, gaseous diffusion technique was popular and had been used extensively for uranium enrichment. | Now-a-days, gas centrifuge technique is used overwhelmingly across the world for uranium enrichment. |
| Gaseous diffusion plants are usually of large size and can process tonnes of fuels in one step. | Gas centrifuge plants can be small scale that can process few tens of kilogram fuel in one step. |
| Owing to its large size, certain activities of these plants can be monitored remotely. Thus these plants are less risky for proliferation perspective. | Gas centrifuge facilities can be operated in in-house mode, and thus it becomes difficult to remotely monitor any activity of such plants. This possesses the risk of nuclear proliferation. |
| Gaseous diffusion plants typically require large amount of electrical power for its operation. | Power consumption per unit mass of enriched uranium is significantly less in gas centrifuge plants. |
References
- SIPRI Yearbook 2007 – Armaments, Disarmament and International Security by Stockholm International Peace Research Institute (Oxford University Press, 2007).
- Uranium Enrichment by United States Nuclear Regulatory Commission, available at https://www.nrc.gov/materials/fuel-cycle-fac/ur-enrichment.html