Nuclear fission is one type of nuclear transmutation where a heavier nucleus, when bombarded with the neutron, splits into two or more lighter neutrons. It is also accompanied by the release of one or more neutrons and thermal energy owing to mass defect. Human has already mastered the nuclear fission reaction, and now-a-days it is extensively used in nuclear reactors for electricity or power generation and also in nuclear weapons. In nuclear reactors, fission is initiated by either thermal neutrons (0.025eV energy corresponding to the velocity of 2.2 km/s) or fast neutrons (1 – 10MeV energy corresponding to the velocity of about 5×104 km/s). Prompt neutrons that are released as fission products can also initiate fission of other intact isotopes available within the fuel, which ultimately leads to the formation of much desirable self-sustained chain reaction. However, every radioactive isotope cannot sustain chain reaction. Thus any radioactive isotope cannot be used as nuclear fuel. Only three isotopes are considered chemically and economically suitable for this purpose, namely Uranium-233, Uranium-235 and Plutonium-239. These three are thus termed as fissile isotopes. Among these three, U-235 is the only naturally available one. However, its availability is very low (only about 0.7%) as natural uranium mostly contains Uranium-238 (about 99.3%).
Even though U-238 is abundantly available on Earth, it is not one fissile isotope as it fails to sustain the much desired chain reaction, and thus it cannot be used as nuclear fuel. Hence the proportion of U-235 within the natural uranium is required to increase artificially for the use as fuel in nuclear reactors. Such a process of increasing percentage of U-235 is called uranium enrichment. Typically, thermal reactors require 3 – 5% enriched fuel (except PHWR where no enrichment is usually required as it uses heavy water as coolant-cum-moderator), while fast breeder reactors require 15 – 20% enriched fuel. Above 90% enrichment is necessary for weapon grade uranium fuel. Whatever be the enrichment, only U-235 isotopes participate in fission reaction. Accordingly, only a tiny portion of the uranium fuel is utilized for heat generation in the nuclear reactors, while majority of the fuel remains unutilized. Thus the uranium fuel for reactors in the nuclear power plant contains both U-238 and U-235 isotopes in different proportions based on the enrichment, but only U-235 undergoes nuclear fission to generate necessary thermal energy. Various similarities and differences between Uranium-235 and Uranium-238 isotopes are given below in table format.
Similarities between U-235 and U-238
- U-235 and U-238 are two isotopes of uranium. Both contain same number of electrons and protons (92 each).
- Both the isotopes are naturally available; however, their abundancy has wide gap. While about 99.28% of the entire Earth’s uranium is basically U-238, only a meagre 0.72% is U-235.
- Both the isotopes are radioactive, although they have different half-lives.
- Nuclear fuel that is used in reactors of the nuclear power plant contains both the isotopes in varying proportions. However, only U-235 participated in fission, while the other one remains intact.
Differences between U-235 and U-238
| Uranium-235 | Uranium-238 |
|---|---|
| An electrically neutral uranium-235 isotope contains 92 electrons, 92 protons and 143 neutrons (i.e. e = 92, p = 92, n = 143). So its atomic number is 92 and mass number is 235. | An electrically neutral uranium-238 isotope contains 92 electrons, 92 protons and 146 neutrons (i.e. e = 92, p = 92, n = 146). So its atomic number is 92 and mass number is 238. |
| Even though uranium is abundantly available on Earth, the uranium-235 isotope has low abundancy (only about 0.72% of the entire Earth’s uranium is U-235). | Uranium-238 isotope is most common isotope of uranium found on Earth (about 99.28% of the entire Earth’s uranium is basically U-238). |
| Its half-life (t1/2) is approximately 0.7038 ´ 109 years. | Its half-life (t1/2) is approximately 4.468 ´ 109 years. |
| Its atomic mass is about 235.043u. It is slightly lighter than U-238. This mass difference is used for enriching purposes (gaseous diffusion and gas centrifuge). | It is slightly heavier than U-235. Its atomic mass is about 238.05u. |
| It has higher probability of alpha-decay and thus it is less stable. | Chance of alpha-decay is less for U-238 and thus it is more stable. |
| Owing to spontaneous disintegration (natural radioactivity), U-235 undergoes alpha-decay to produce thorium-231 isotope (90Th231). | U-238 also undergoes alpha-decay spontaneously but produces thorium-234 isotope (90Th234). |
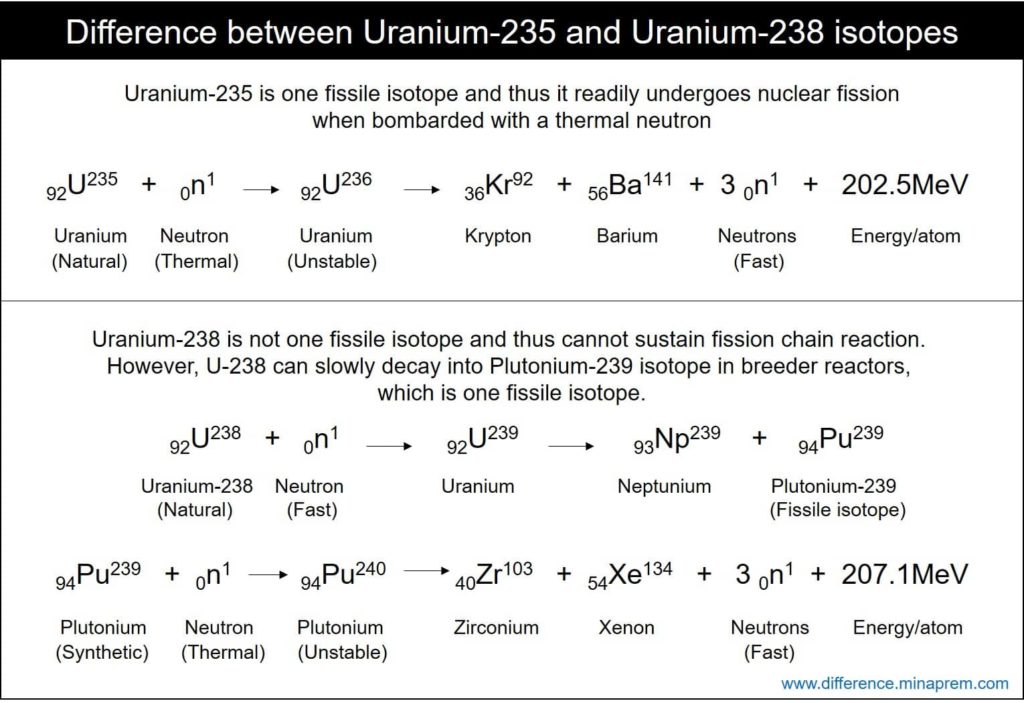
| Uranium-235 is one fissile isotope, thus it can sustain nuclear fission chain reaction with thermal neutron. | Uranium-238 is not a fissile isotope and thus cannot sustain chain reaction with any neutron. However, U-238 is one fertile material and thus it can produce Pu-239 (one fissile isotope) by nuclear breeding in fast breeder reactor. |
| Its fission cross-section with thermal neutron is around 583 barns, and that with fast neutron is about 1 barn. | Its fission cross-section with thermal neutron is as low as 0.00002 barn, and that with fast neutron is about 0.3 barn. |
| It is the active constituent of nuclear fuel. Thus natural uranium is enriched with U-235 (3 – 5% enrichment is required for light water thermal reactors, 15 – 20% enrichment is required for fast reactors, while about 90% enrichment is required for nuclear weapon). Only U-235 isotopes in the entire fuel undergo nuclear fission to liberate heat energy. | Even though a significant part of the nuclear fuel is made up of U-238, mostly it does not participate in nuclear fission reaction and thus it is not the active part of nuclear fuel. In thermal reactors, entire U-238 remains intact after complete fuel burn-up. However, a small portion of U-238 can breed into Pu-239 in fast breeder reactors, and this Pu-239 can participate in nuclear fission to generate heat. |
| In nuclear fission, when an atom of uranium-235 splits into krypton and barium, it liberates 202.5MeV energy (arises from mass defect in reaction). | It usually does not undergo nuclear fission reaction to liberate energy because of its very low fission cross-section. |
References
- Uranium Enrichment by World Nuclear Association, available at https://www.world-nuclear.org/information-library/nuclear-fuel-cycle/conversion-enrichment-and-fabrication/uranium-enrichment.aspx
- Nuclear Energy – An Introduction to the Concepts, Systems, and Applications of Nuclear Processes (2015) by R. L. Murray and K. E. Holbert (Butterworth-Heinemann). https://doi.org/10.1016/C2012-0-02697-X
- Storage and Hybridization of Nuclear Energy – Techno-economic Integration of Renewable and Nuclear Energy (2019) by H. Bindra and S. Revankar (Academic Press). https://doi.org/10.1016/C2017-0-00346-4