Oxy-fuel gas welding is one fusion welding process where components are permanently joined together. Here heat is supplied by burning a suitable gaseous carbonaceous fuel with oxygen. Potential fuel oxy-fuel gas welding incudes acetylene, propylene, propane, MAPP (methylacetylene-propadiene propane) gas, and natural gas; however, acetylene (C2H2) is frequently used as it offers maximum flame temperature. Both the fuel and oxygen are stored separately in cylinders, and these are mixed in a torch before delivering the mixture through a nozzle. This mixture is then ignited to produce a flame. The exothermic reaction of combustion between the acetylene and oxygen produces heat. This heat is used to melt down the faying surfaces of the base plates and filler metal in order to form a weld bead. A fixed volume (or mass) of oxygen is always desired for complete combustion of per unit volume (or mass) of a specific fuel. The required volume / mass of oxygen for a given fuel can be theoretically obtained by stoichiometric analysis. For combustion of acetylene in oxygen, the stoichiometric oxygen-fuel ratio is 13.26 : 1 (based on mass) or 11.92 : 1 (based on volume). It indicates that 13.26 kg of oxygen is required for complete combustion of 1 kg of acetylene. Alternatively, 11.92 m3 of oxygen is required for complete combustion of 1 m3 of acetylene.
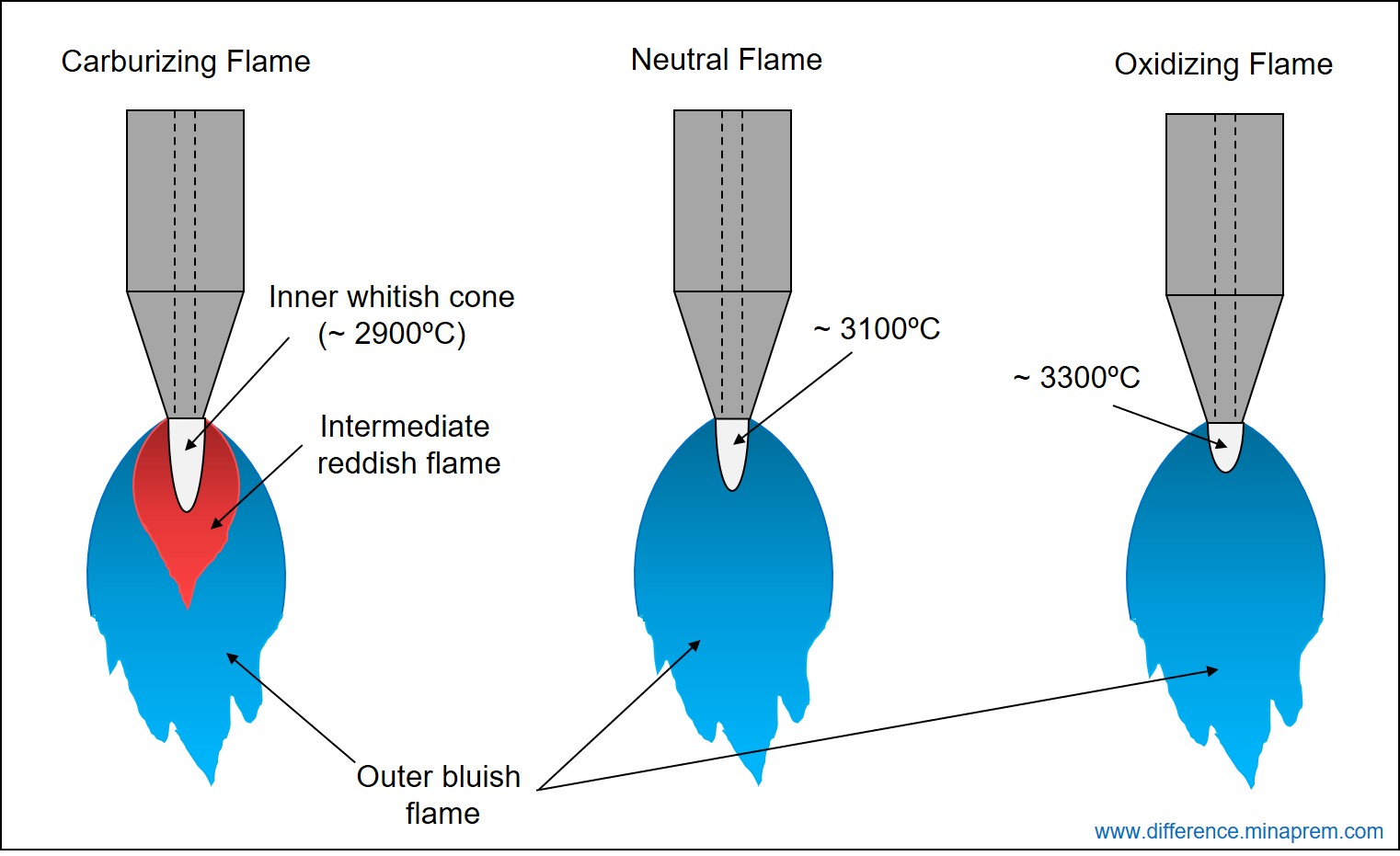
In oxy-acetylene gas welding, if oxygen-acetylene mixture is stoichiometric, then the resulting flame is a neutral flame. Here, entire acetylene and oxygen supplied through nozzle react with each other to produce carbon dioxide and water vapour, and no acetylene or oxygen remains residue after the combustion. Similarly, if more acetylene is supplied than that is required stoichiometrically, then certain amount of acetylene will remain left as residue. The resulting flame is known as Carburizing Flame or Reducing Flame. As acetylene is a carbonaceous element, so the excess acetylene can react with the oxygen elements present within the molten weld bead. This can result in a hard and brittle weld bead. On the other hand, if more oxygen is supplied than that is required stoichiometrically, then certain amount of oxygen will remain left even after complete combustion of entire acetylene. The resulting flame is called Oxidizing Flame, as the excess oxygen can further oxidize the elements of molten weld bead. Typical appearance of three types of flame is shown above. Various similarities and differences between carburizing or reducing flame and oxidizing flame are given below in table format.
Similarities between reducing flame and oxidizing flame
- Irrespective of flame type, supply of gaseous carbonaceous fuel and oxygen are indispensably necessary. Based on the relative flow rate of fuel and oxygen, the flame type changes.
- Combustion between fuel and oxygen takes place in both types of flame. Though the completeness of combustion varies for different types of flame.
- An inner whitish cone forms just at the nozzle exit regardless of the flame type. However, the size, temperature and heat intensity in this inner cone vary based on the flame type.
- Most industrial gas welding torches have the provision to separately manipulate the supply rate of acetylene and oxygen. Thus, a reducing flame can be easily converted to neutral or oxidizing flame by adjusting the valves even during welding, or vice versa.
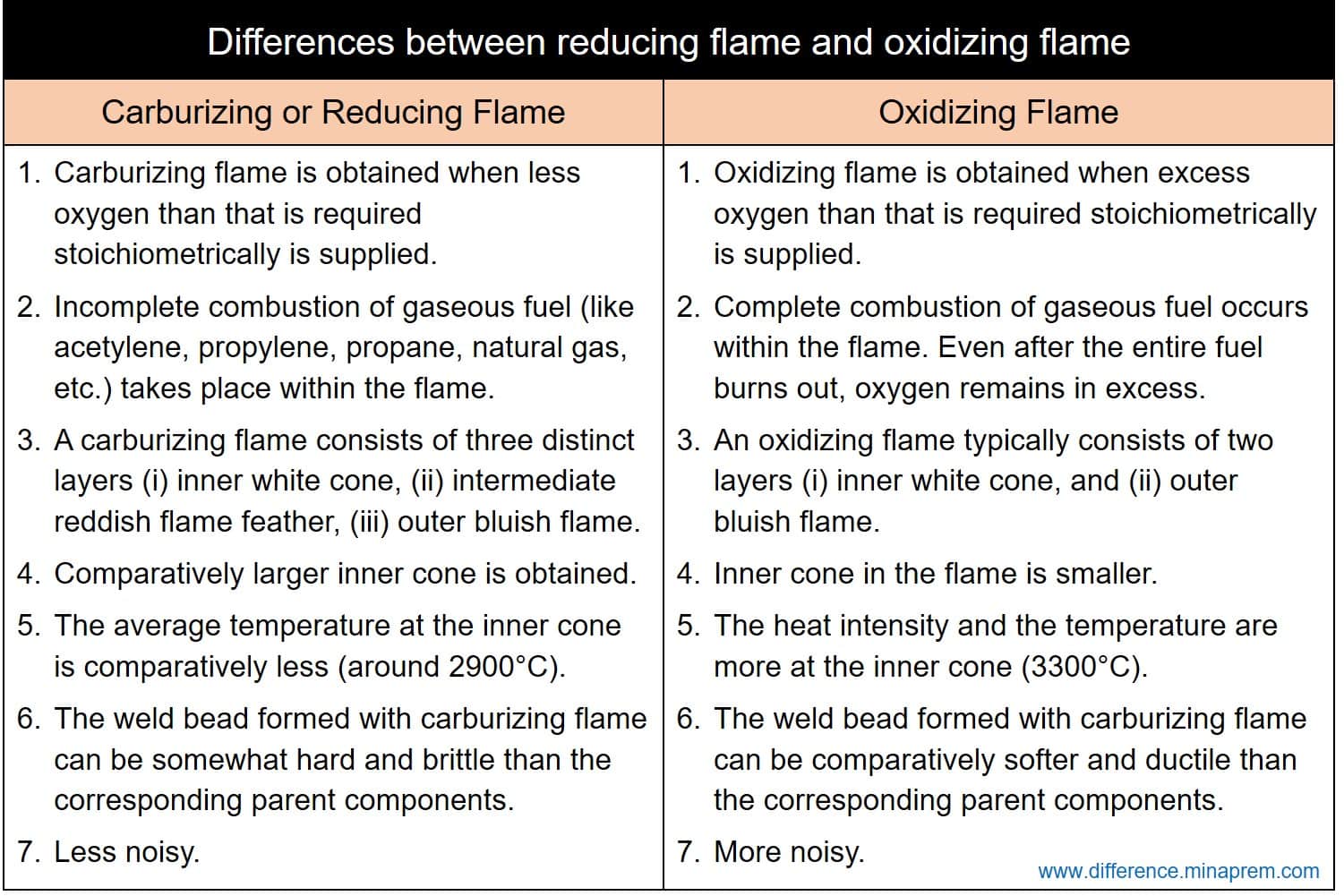
Differences between reducing flame and oxidizing flame
| Carburizing or Reducing Flame | Oxidizing Flame |
|---|---|
| Carburizing flame is obtained when less oxygen than that is required for stoichiometrically complete combustion is supplied. | Oxidizing flame is obtained when excess oxygen than that is required for stoichiometrically complete combustion is supplied. |
| Incomplete combustion of gaseous fuel (like acetylene, propylene, propane, natural gas, etc.) takes place within the flame. | Complete combustion of gaseous fuel occurs within the flame. Even after the entire fuel burns out, oxygen remains in excess. |
| A carburizing flame consists of three distinct layers (i) inner white cone, (ii) intermediate reddish flame feather, and (iii) outer bluish flame. | An oxidizing flame typically consists of two layers (i) inner white cone, and (ii) outer bluish flame. |
| Due to scarcity in oxygen, first stage of combustion reaction (acetylene to carbon monoxide) occurs for a wider area. This results to a comparatively larger inner cone. | Due to excess oxygen supply, first stage of combustion reaction occurs quickly within a small area. This results in a smaller inner cone. |
| Due to larger size, the average temperature at the inner cone is comparatively less (around 2900°C). The heat intensity is also comparatively less at the inner cone. | Due to smaller size, the heat intensity and the temperature are more at the inner cone. The inner cone temperature can be as high as 3300°C. |
| Carburizing flame can (i) induce carbon atoms into the weld bead, or (ii) take out oxygen atoms from the weld bead. | Oxidizing flame can (i) diffuse oxygen atoms into the weld bead, or (ii) take out carbon atoms from the weld bead. |
| The weld bead formed with carburizing flame can be somewhat hard and brittle than the corresponding parent components. | The weld bead formed with carburizing flame can be comparatively softer and ductile than the corresponding parent components. |
| Gas welding with this flame is less noisy. | Gas welding with this flame can be comparatively more noisy. |
| Carburizing flame can be used when components are rich in carbon or free from oxygen. This flame is commonly used while joining high carbon steel, cast iron, high speed steel, oxygen-free copper, etc. | Oxidizing flame is preferred for joining components made of low-carbon ferrous alloys and non-ferrous alloys. |
References
- Manufacturing Technology: Foundry, Forming and Welding by P. N. Rao (Tata McGraw Hill Education Private Limited).
- A Text-Book of Welding Technology by O. P. Khanna (Dhanpat Rai Publications).