Thermodynamic system is defined as a definite quantity of matter or a region in space upon which attention is concentrated for the analysis of a problem. Everything in this universe external to the system is called surroundings. The system is separated from the surroundings by the system boundary. Every interaction between the system and surroundings occurs across the boundary. There are two distinct ways of interaction between system and surroundings, as enlisted below.
- Mass interaction
- Energy interaction (like heat, work, etc.)
It is worth mentioning here that energy interaction can be in different forms like heat transfer, work transfer, electrical energy transfer, etc. The system boundary sometimes permit either both types of interaction or only one type of interaction or no interaction. Based on the interaction scenario between the system and surroundings, thermodynamic systems can be classified into three categories, as enlisted below.
- Open system
- Closed system
- Isolated system
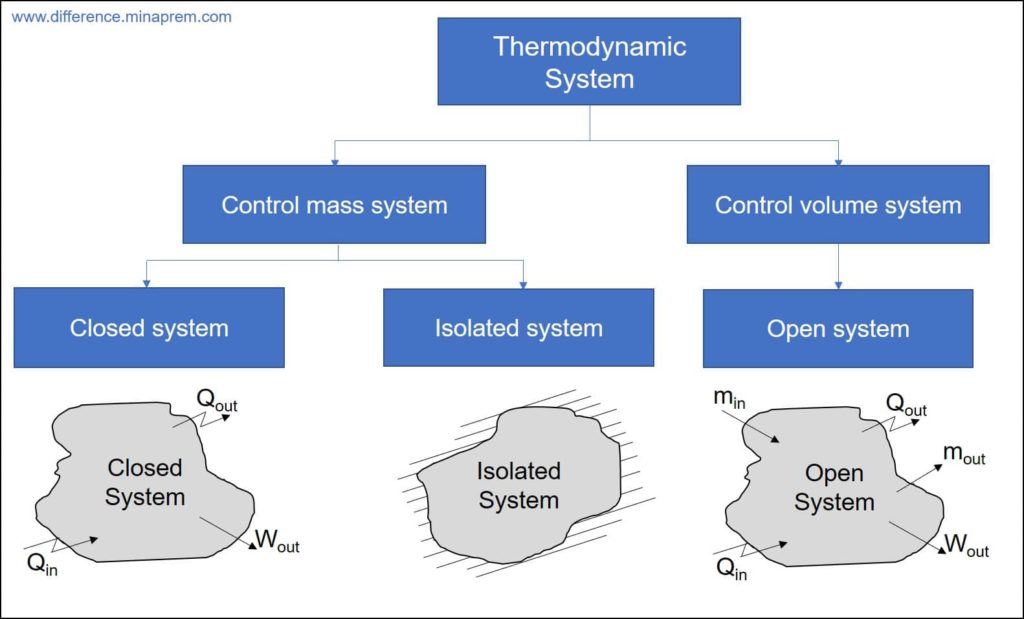
Open system and its example
All such thermodynamic systems where both the mass interaction and energy interaction occur between the concerned system and its surroundings are called open systems.
Examples of open system: Boiler, Nuclear reactor, Combustion chamber, Turbine, Condenser, Pump, Heat exchanger, etc.
Closed system and its example
All such thermodynamic systems where only energy interaction occurs between the concerned system and its surroundings are called closed systems. Thus, no mass interaction occurs between a closed system and its surroundings.
Examples of closed system: Refrigerant or working fluid of refrigerator unit, Coolant of nuclear PWR or PHWR, Hot water kept inside a PETE bottle, etc. can be considered as closed system for practical cases.
Isolated system and its example
All such thermodynamic systems where neither mass interaction nor energy interaction occurs between the concerned system and its surroundings are called isolated systems.
Examples of isolated system: A perfect isolated system does not exist as energy interaction in the form of heat radiation will always occur so long as there exist temperature difference between system and surroundings. Practically, the matter inside a flask having inbuild radiation shields can be considered as isolated system.