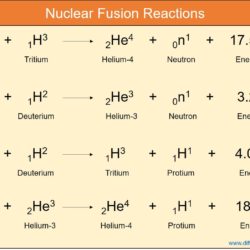
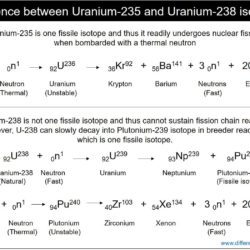
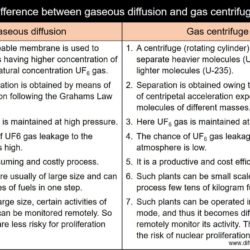
Difference Between Thermal Neutron and Fast Neutron
Neutron is a sub-atomic particle situated within the nucleus of the atom. It is electrically neutral (i.e. chargeless particle) and has mass slightly higher than that of the proton. Neutrons, together with protons, are called nucleons. When the neutron stays within the nucleus, it remains highly stable and can be expelled by nuclear transmutation only. However, when a neutron stays outside the nucleus, it becomes highly unstable and undergoes radioactive