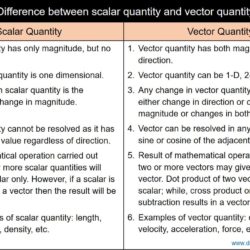
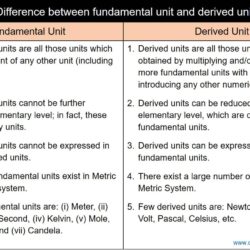
Difference Between Scalar Quantity and Vector Quantity
Every scientific explanation is realized with the aid of a number of physical quantities — each expresses a special meaning and significance in that context. By definition, a physical quantity is measurable and quantifiable physical property that carries unique information with it. Based on the dependency nature, such quantities can be of two types — fundamental and derived. A fundamental quantity is one that is independent of other properties; while,