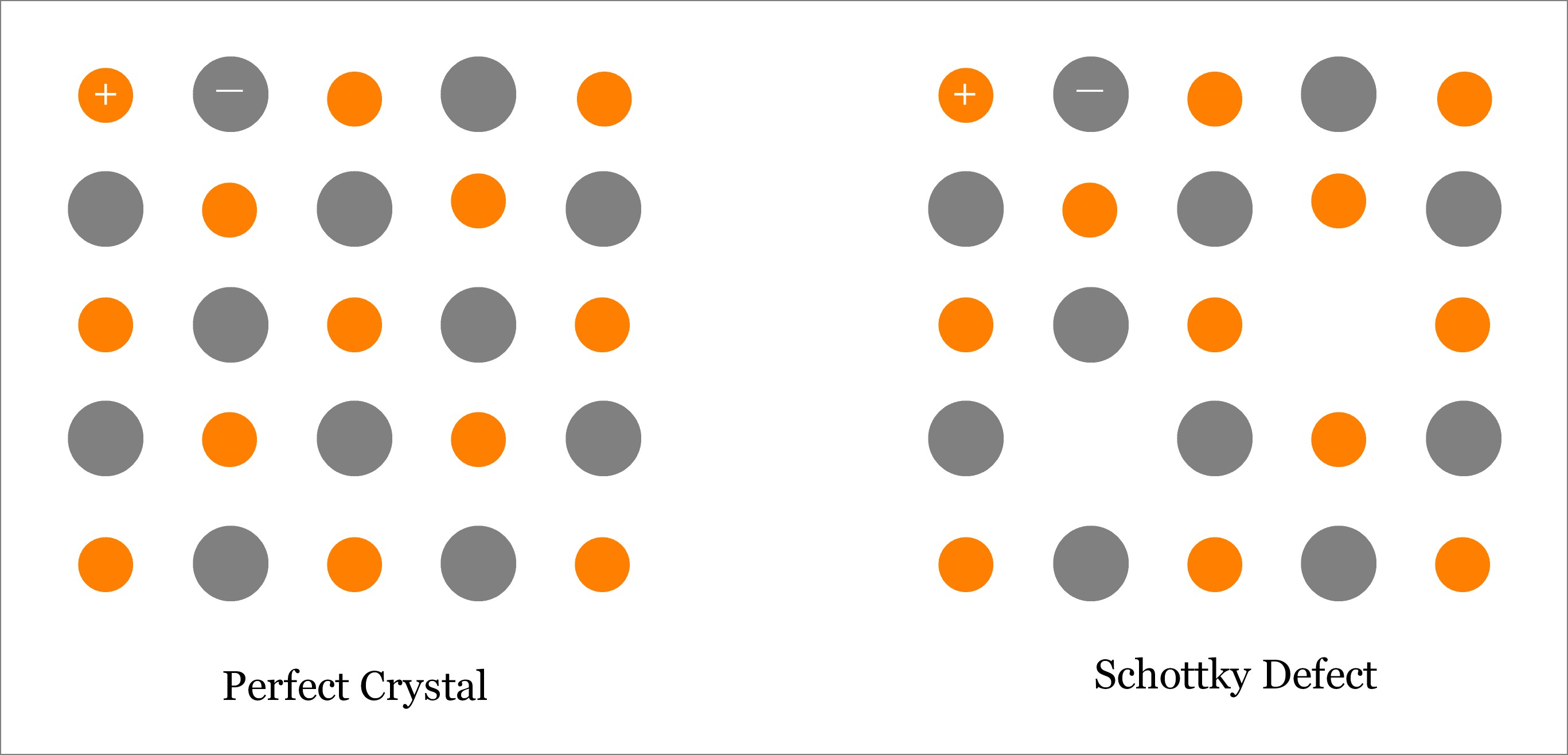
Schottky defect occurs when oppositely charged atoms (cation and anion) leave their corresponding lattice sites and create a pair of Vacancy Defects. Since both cation and anion leave the lattice sites at the same time, so overall electrical neutrality of the crystal is maintained; however, density reduces because of the vacancies. Schottky defects occur in ionic crystals where the size of anion is almost same with the size of the cation. Read more about Schottky defect.
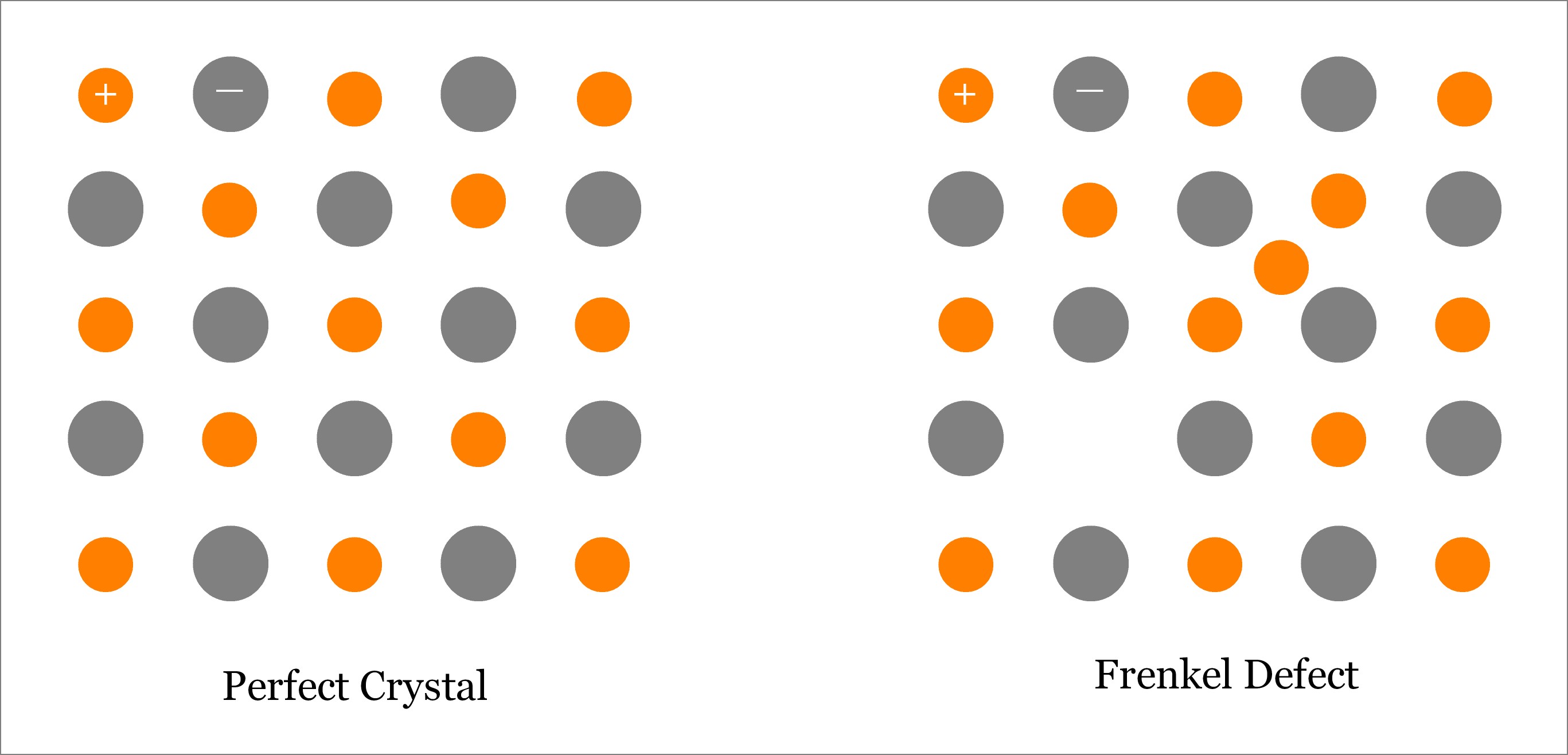
Frenkel Defect is one type of point defect where an atom (better to say ion, especially cation) leaves its original lattice site and occupies an interstitial position on the same crystal. Usually, this type of defect is observed in ionic solids, where size of anion is substantially larger than the size of cation. Read more about Frenkel defect.
Although both Schottky defect and Frenkel defect are point defects and occur only in ionic materials, there exists a big margin between them. Differences between Schottky defect and Frenkel defect are portrayed in the following table.
| Schottky Defect | Frenkel Defect |
|---|---|
| Schottky defect occurs in those ionic crystals where difference in size between cation and anion is small. | Frenkel defect usually occurs in those ionic crystals where size of anion is quite large as compared to that of the cation. |
| In Schottky defect, both cation and anion leave the solid crystal. | In Frenkel defect, only the smaller ion (cation) leaves its original lattice site; whereas, the anion remains in original lattice sites. |
| The atoms permanently leave the crystal. | Here, atoms leave the original lattice site and occupy interstitial position. So atoms reside within the solid crystal. |
| One Schottky defect leads to the formation of two vacancies. | One Frenkel defect creates one vacancy and one self-interstitial defect. |
| Two atoms reduce from the crystal for each Schottky defect. | The number of atoms present in the crystal before and after Frenkel defect remains same. |
| Due to vacancy formation, Schottky defect reduces density of the solid. | Density of the solid crystal before and after Frenkel defect remains same as no atom leaves the solid. |
Common materials where Schottky defect can be found are:
|
Common materials where Frenkel defect can be found are:
|
References
- Book: Callister’S Materials Science and Engineering by R. Balasubramaniam (Wiley India Private Limited). Buy this book
- Book: Materials Science and Engineering by V. Raghavan (PHI Learning Private Limited). Buy this book
- Book: Materials Science and Engineering: Problems with Solutions by M. N. Shetty (PHI Learning Private Limited). Buy this book